Search Thermo Fisher Scientific
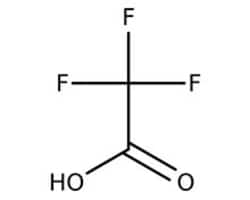
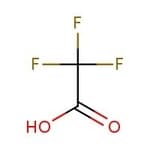
Trifluoroacetic Acid
Trifluoracetic acid is an organofluorine compound that is a structural analogue of acetic acid with all three of the acetyl group’s hydrogen atoms replaced by fluorine atoms. It is stronger than acetic acid, having an acid ionization constant of pKA 0.23. Trifluoracetic acid is available in various quantities and reagent grades. It is widely used in organic synthesis, mass spectrometry, and NMR spectroscopy.
Products (4)
Learn More (0)
Documents & Support
(292)4 Products
Filter
C2HF3O2, CAS Number-76-05-1, acetic acid, trifluoro, cf3cooh, kyselina trifluoroctova, perfluoroacetic acid, trifluoroacetic acid, trifluoroaceticacid, trifluoro acetic acid, trifluoro-acetic acid, trifluoroacetic acid, trifluoroethanoic acid, 100mL, 72 deg.C, CHEBI:45892, Colorless, 114.02g/mol
Trifluoroacetic Acid, for DNA Synthesis And Protein Sequencing
Trifluoroacetic Acid, >-99.5%, C2HF3O2, CAS Number-76-05-1, trifluoroethanoic acid, trifluoroacetic acid, cf3cooh, acetic acid, trifluoro, trifluoro-acetic acid, perfluoroacetic acid, kyselina trifluoroctova, trifluoro acetic acid, trifluoroacetic acid, trifluoroaceticacid, 50mL
The combination of properties like solubility in most of the solvents, volatility, catalytic property and strong acidity with non-oxidizing nature makes it a widely used reagent in an organic synthesis.