Search Thermo Fisher Scientific
Thermo Scientific Chemicals
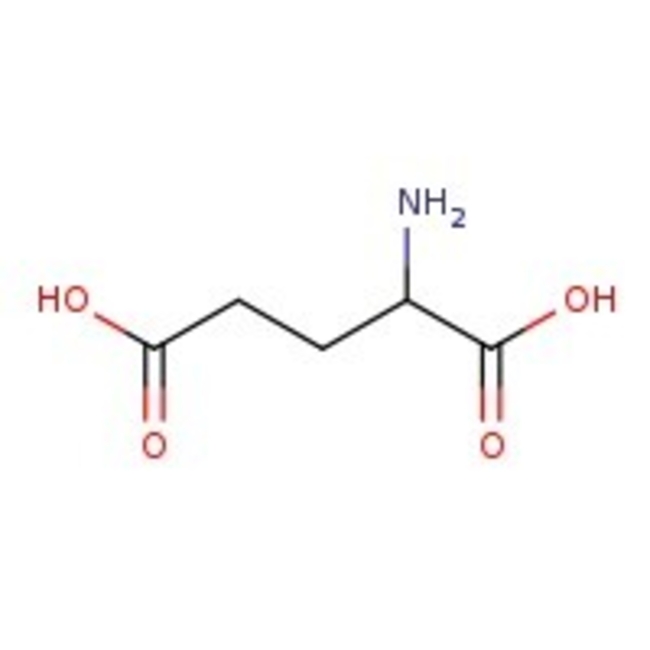
L-Glutamic acid, 99%
CAS: 56-86-0 | C5H9NO4 | 147.13 g/mol
| Catalog Number | Quantity |
|---|---|
| FSKA15031.30 | 250 g |
Catalog number FSKA15031.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialL-Glutamic Acid
Melting Point189°C to 192°C (decomposition)
CAS56-86-0
Health Hazard 1H500
Recommended StorageAmbient temperatures
View more
It is used as a chiral building-block in the synthesis of the (S)-isomer of -butyrolactone-4-carboxylic acid. L-Glutamic acid is widely used in fields of medicine, food processing, industry, etc.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is used as a chiral building-block in the synthesis of the (S)-isomer of -butyrolactone-4-carboxylic acid. L-Glutamic acid is widely used in fields of medicine, food processing, industry, etc.
Solubility
It is very slightly soluble in cold water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents. Stable under recommended storage conditions.
It is used as a chiral building-block in the synthesis of the (S)-isomer of -butyrolactone-4-carboxylic acid. L-Glutamic acid is widely used in fields of medicine, food processing, industry, etc.
Solubility
It is very slightly soluble in cold water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from strong oxidizing agents. Stable under recommended storage conditions.
RUO – Research Use Only
General References:
- Watkins.; Evans. Excitatory amino acid transmitters. Annu.Rev.Pharmacol.Toxicol. 1981, 165, (21), 354-362.
- Monaghan et al . The excitatory amino acid receptors: their classes, pharmacology and distinct properties in the function of the central nervous system. Annu.Rev.Pharmacol.Toxicol. 1989, 365, (29), 382-385.
- For use as a chiral building-block in the synthesis of the (S)-isomer of -butyrolactone-4-carboxylic acid, see: Org. Synth. Coll., 7, 99 (1990):
- For the differential protection of the ɑ-carboxyl group of N-protected glutamic acid by reaction with formaldehyde to form the cyclic 5-oxazolidinone, see: Synthesis, 542 (1989).