Search Thermo Fisher Scientific
Thermo Scientific Chemicals
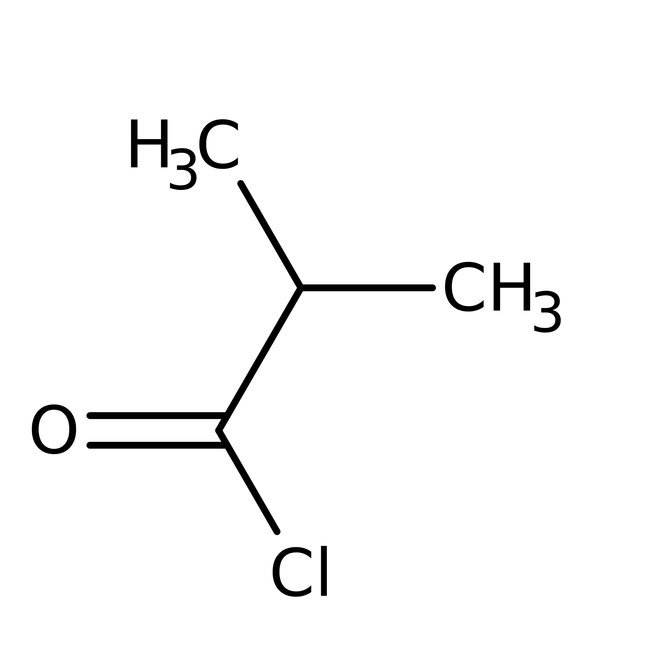
Isobutyryl chloride, 98%
CAS: 79-30-1 | C4H7ClO | 106.549 g/mol
| Catalog Number | Quantity |
|---|---|
| FSKB24472.22 | 100 g |
Catalog number FSKB24472.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialIsobutyryl chloride
CAS79-30-1
Health Hazard 1H225-H290-H302-H314-H330
Health Hazard 2GHS H Statement
H225-H314
H225-H314
Health Hazard 3P210-P233-P234-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P280-P284-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P390-P501c
View more
Isobutyryl chloride is used in the preparation of fully isobutyramide terminated hyperbranched polyethylenimines. It plays an important role in the separation and identification of shikonin and its ester derivatives in the root epidermis of Echium italicum L. It acts as an acylating agent for the ketimine derivatives of alfa-amino esters, nucleosides and pyrroles. Further, it is used in the preparation of organic peroxides. In addition to this, it is used as an intermediate for dyes, textile auxiliaries and peroxide compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Isobutyryl chloride is used in the preparation of fully isobutyramide terminated hyperbranched polyethylenimines. It plays an important role in the separation and identification of shikonin and its ester derivatives in the root epidermis of Echium italicum L. It acts as an acylating agent for the ketimine derivatives of alfa-amino esters, nucleosides and pyrroles. Further, it is used in the preparation of organic peroxides. In addition to this, it is used as an intermediate for dyes, textile auxiliaries and peroxide compounds.
Solubility
Miscible with chloroform, glacial acetic acid, ether, toluene, methylene chloride and benzene.
Notes
Moisture sensitive. Incompatible with bases, water, strong oxidizing agents and alcohols.
Isobutyryl chloride is used in the preparation of fully isobutyramide terminated hyperbranched polyethylenimines. It plays an important role in the separation and identification of shikonin and its ester derivatives in the root epidermis of Echium italicum L. It acts as an acylating agent for the ketimine derivatives of alfa-amino esters, nucleosides and pyrroles. Further, it is used in the preparation of organic peroxides. In addition to this, it is used as an intermediate for dyes, textile auxiliaries and peroxide compounds.
Solubility
Miscible with chloroform, glacial acetic acid, ether, toluene, methylene chloride and benzene.
Notes
Moisture sensitive. Incompatible with bases, water, strong oxidizing agents and alcohols.
RUO – Research Use Only
General References:
- Sparling, B. A.; Tucker, J. K.; Moebius, D. C.; Shair, M. D. Total Synthesis of (-)-Nemorosone and (+)-Secohyperforin. Org. Lett. 2015, 17 (14), 3398-3401.
- Lopchuk, J. M.; Gribble, G. W. Total synthesis of atorvastatin via a late-stage, regioselective 1,3-dipolar münchnone cycloaddition. Tetrahedron Lett. 2015, 56 (23), 3208-3211.