Search Thermo Fisher Scientific
Thermo Scientific Chemicals
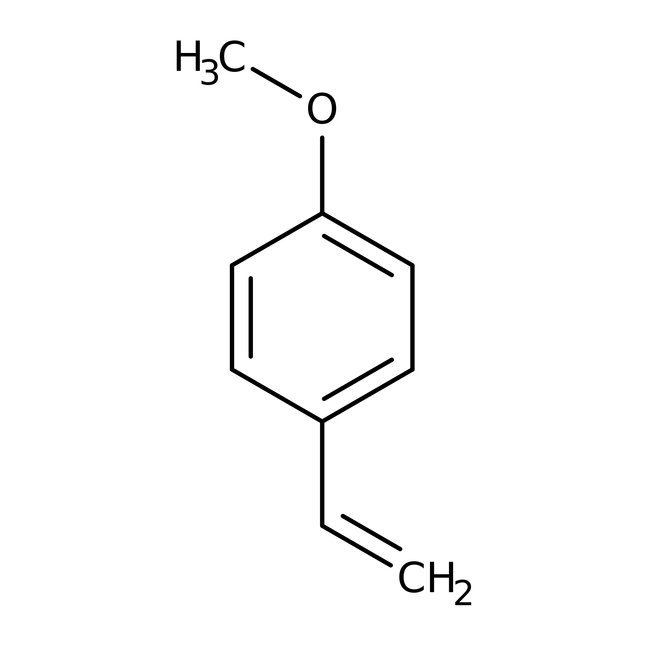
4-Methoxystyrene, 98%, stab. with 0.1% 4-tert-butylcatechol
CAS: 637-69-4 | C9H10O | 134.18 g/mol
| Catalog Number | Quantity |
|---|---|
| FSKB22353.22 | 100 g |
Catalog number FSKB22353.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material4-Methoxystyrene
Name Notestabilized with 0.1% 4-tert-butylcatechol
CAS637-69-4
Health Hazard 1H227-H315-H320
Health Hazard 2GHS H Statement
H227
Combustible liquid.
H227
Combustible liquid.
View more
4-Methoxystyrene is employed in the ferric chloride-catalyzed addition of activated methylenes to styrenes. It acts as a monomer in polymerization reactions. Further, it is used to prepare 1,1,2,2-Tetracyano-3-(p-methoxyphenyl)cyclobutane by reacting with ethenetetracarbonitrile.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Methoxystyrene is employed in the ferric chloride-catalyzed addition of activated methylenes to styrenes. It acts as a monomer in polymerization reactions. Further, it is used to prepare 1,1,2,2-Tetracyano-3-(p-methoxyphenyl)cyclobutane by reacting with ethenetetracarbonitrile.
Solubility
Slightly miscible with methanol.
Notes
Light sensitive. Store in cool place. Incompatible with strong oxidizing agents and strong acids.
4-Methoxystyrene is employed in the ferric chloride-catalyzed addition of activated methylenes to styrenes. It acts as a monomer in polymerization reactions. Further, it is used to prepare 1,1,2,2-Tetracyano-3-(p-methoxyphenyl)cyclobutane by reacting with ethenetetracarbonitrile.
Solubility
Slightly miscible with methanol.
Notes
Light sensitive. Store in cool place. Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Perkowski, A. J.; You, W.; Nicewicz, D. A. Visible Light Photoinitiated Metal-Free Living Cationic Polymerization of 4-Methoxystyrene. J. Am. Chem. Soc. 2015, 137 (24), 7580-7583.
- Miyazawa, K.; Koike, T.; Akita, M. Regiospecific Intermolecular Aminohydroxylation of Olefins by Photoredox Catalysis. Chem. Eur. J. 2015, 21 (33), 11677-11680.