Search Thermo Fisher Scientific
Thermo Scientific Chemicals
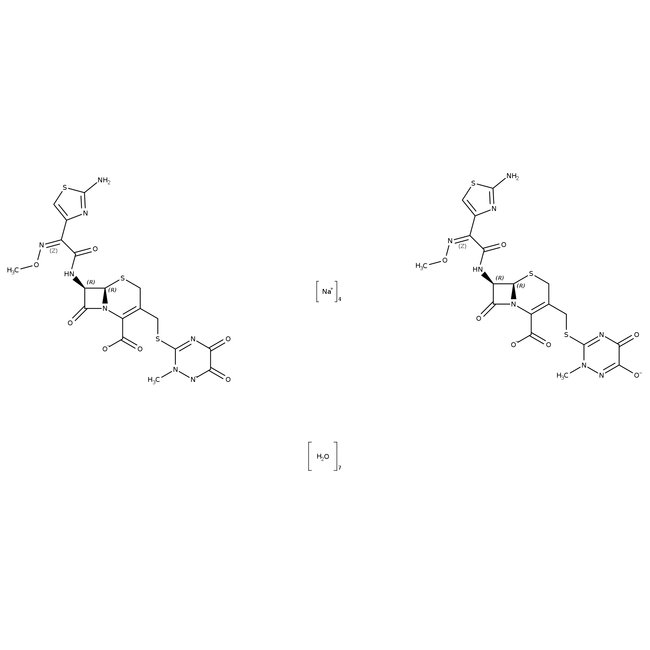
Ceftriaxone sodium salt hemiheptahydrate
Ceftriaxone, CAS # 104376-79-6, sodium salt hemiheptahydrate is the sodium salt of ceftriaxone, a third-generation beta-lactam antibiotic of the cephalosporin class.
| Catalog Number | Quantity |
|---|---|
| ACO455420010 | 1 g |
Catalog number ACO455420010
Price (SGD)
253.00
EA
Quantity:
1 g
Price (SGD)
253.00
EA
Specifications
Chemical Name or MaterialCeftriaxone sodium salt hemiheptahydrate
TypeCeftriaxone Sodium Salt Hemiheptahydrate
CAS104376-79-6
Health Hazard 1Danger
Health Hazard 2GHS H Statement Causes skin irritation. May cause an allergic skin reaction. Causes serious eye irritation. May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause respiratory irritation.
View more
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
• Ceftriaxone is the sodium salt of ceftriaxone, a beta-lactam antibiotic of the cephalosporin class that works by inactivating penicillin-binding proteins
Applications
• Penicillin-binding protein inactivation inhibits peptidoglycan cross-linkage and leads to destruction of the cell wall and subsequent lysis
• It exhibits antibiotic activity against gram-negative bacteria
RUO – Research Use Only
General References:
- Grégoire, M.; Dailly, E.; Le Turnier, P.; Garot, D.; Guimard, T.; Bernard, L.; Tattevin, P.; Vandamme, Y.M.; Hoff, J.; Lemaitre, F.; Verdier, M.C.; Deslandes, G.; Bellouard, R.; Sébille, V.; Chiffoleau, A.; Boutoille, D.; Navas, D.; Asseray, N. High-Dose Ceftriaxone for Bacterial Meningitis and Optimization of Administration Scheme Based on Nomogram. Antimicrob Agents Chemother. 2019, 63(9), e00634-19.
- Ross, J.D.C.; Brittain, C.; Cole, M.; Dewsnap, C.; Harding, J.; Hepburn, T.; Jackson, L.; Keogh, M.; Lawrence, T.; Montgomery, A.A.; Roberts, T.E.; Sprange, K.; Tan, W.; Thandi S, White J.; Wilson, J.; Duley, L. G-ToG trial team. Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG): a randomised non-inferiority trial. Lancet. 2019, 22, 393(10190), 2511-2520. Erratum in: Lancet. 2019, Erratum in: Lancet, 394(10205), 1230.