Search Thermo Fisher Scientific
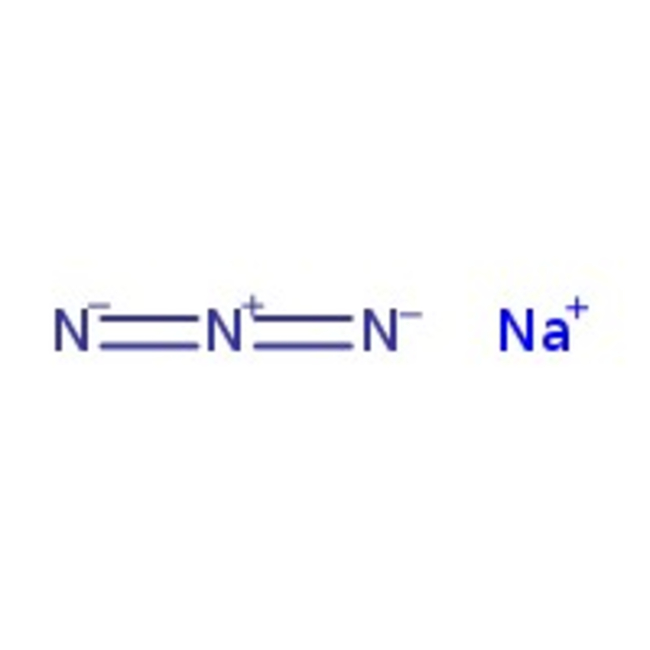
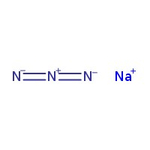
Sodium azide, 99+%, for biochemistry
General Information
• Sodium azide is a precursor for preparing inorganic azides such as lead azide and silver azide
• One mechanism of action associated with this compound can be related to the inhibition of mitochondrial ATPase, that consequently suppresses oxidative phosphorylation
Applications
• Sodium azide is an inorganic compound with antibacterial and mutagenic activities mainly used in biological samples
• Sodium azide inhibits cytochrome oxidase in gram-negative bacteria. However, its antibacterial capacity is more limited in some gram-positive bacteria (streptococci, pneumococci, lactobacilli), presenting intrinsic resistance
• Sodium azide is used for the introduction of an azide group to prepare a desired compound
• It is useful in biochemical approaches as a probe reagent and a preservative
• This compound can be used to synthetize blue fluorescent copolymers and metal phosphonates
General References:
- Abdollahi-Alibeik, M.; Moaddeli, A. Multi-component one-pot reaction of aldehyde, hydroxylamine and sodium azide catalyzed by Cu-MCM-41 nanoparticles: a novel method for the synthesis of 5-substituted 1 H-tetrazole derivatives. New J. Chem. 2015, 39 (3), 2116-2122.
- Zeng, M.; Yang, Y. H.; Li, J. J.; Chen, Y.; Cui, D. M.; Zhang, C. Copper Catalyzed Synthesis of Aryl Azides from Aryl Bromides and Sodium Azide. Asian J. Chem. 2015, 27 (5), 1698-1700.